Release time :2024-04-07
Source:support@yingchitech.com
Scan:836
Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd
Professor Yan Min's team from the Department of Anesthesiology, the Second Affiliated Hospital of Zhejiang University School of Medicine, together with Associate Professor Che Xianwei's team from Hangzhou Normal University, have published their latest research results in “PAIN”, a leading journal in the field of pain.
This study investigates the opioid peptide analgesic mechanism of prolonged continuous theta burst stimulation (pcTBS). The study was a randomized, double-blind, sham-controlled study to reveal whether the endogenous opioid system is involved in the analgesic effects induced by stimulation of the primary motor cortex (M1) and dorsal lateral prefrontal cortex (DLPFC) by intravenous administration of naloxone, an antagonist of the opioid receptor. The results showed that naloxone reversed the analgesic effects induced by stimulation of the primary motor cortex (M1) and dorsal lateral prefrontal cortex (DLPFC).
The results showed that naloxone reversed the analgesic effects induced by M1-pcTBS and DLPFC-pcTBS. However, only M1-targeted analgesic effects were restored after completion of naloxone metabolism. In contrast to M1, DLPFC targets required two pcTBS to produce significant analgesic effects. In addition, M1-pcTBS and DLPFC-pcTBS selectively increased plasma concentrations of β-endorphin or enkephalin, respectively. Through a strict 'inhibition-reactivation' paradigm, this study confirms the involvement of the endogenous opioid system in M1- and DLPFC-induced analgesia and the existence of dose-dependent effects at different targets. The following is a detailed description.

Repetitive transcranial magnetic stimulation (rTMS) acting on the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) effectively relieves chronic neuropathic pain and migraine, respectively, and its analgesic effect has received widespread attention, but the mechanism of action is still unclear. Therefore, clarifying the analgesic mechanism of rTMS is an important direction to optimize its clinical effects.
Endogenous opioid peptides can inhibit injurious sensory signaling; however, only a few studies have explored the mechanism of endogenous opioid system involved in rTMS analgesia and reported inconsistent results. The three studies that have been conducted differed significantly in terms of stimulation dose. On the other hand, the early studies only targeted the opioid peptide receptor for 'inhibition' and did not use the more rigorous 'inhibition-reactivation' research paradigm. This paradigm could provide stronger causal evidence for drug or intervention studies.
To address these two questions, a two-stage experiment was designed in this study. We first explored whether intravenous naloxone could reverse the analgesic effects of M1-rTMS and DLPFC-rTMS, and then performed a second rTMS stimulation to restore the analgesic effects of rTMS after the metabolism of naloxone was completed. rTMS stimulation was performed by the FDA-approved M-100 Ultimate (Yingchi Technology).
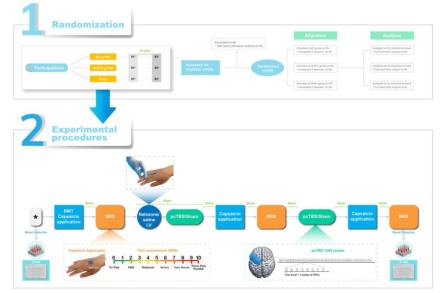
A total of 45 subjects were enrolled and randomized into three groups (M1, DLPFC, Sham). Volunteers visited the laboratory twice (≥2 weeks apart), with one slow intravenous injection of naloxone and the other with an equal dose of saline. The investigators assessed the volunteers' pain and blood indices before the start of the trial (baseline), 30 minutes after the first pcTBS intervention (Post1), and 30 minutes after the second pcTBS intervention (Post2).
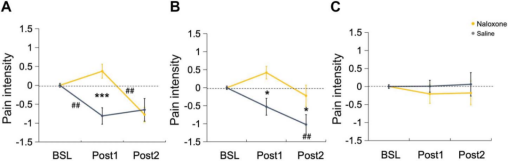
The results of the study showed that the first M1-pcTBS intervention (Post1) reduced pain intensity in the saline condition, whereas two pcTBS (Post2) did not produce a stronger analgesic effect. In the naloxone condition, pain intensity was unchanged after the first M1-pcTBS intervention, and when naloxone was metabolized (Post2), M1-pcTBS significantly reduced pain intensity. In addition, naloxone Post1 pain intensity was significantly higher than saline. For DLPFC targets, two DLPFC-pcTBS interventions (Post2) were required to reduce pain intensity in the saline condition. In the naloxone condition, pain intensity was unchanged after the first DLPFC-pcTBS intervention, whereas after naloxone metabolism (Post2), the second DLPFC-pcTBS still failed to significantly reduce pain, but both naloxone-conditioned Post1 and Post2 pain intensities were significantly higher than saline. no significant differences were seen in the Sham group for any of the conditions.
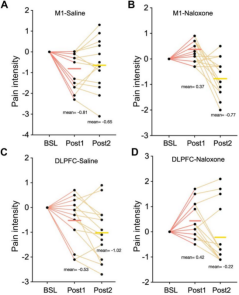
Individual data on changes in pain under saline and naloxone conditions in the M1 and DLPFC groups are shown below. Two DLPFC stimulations produced a superimposed analgesic effect, whereas a single M1 stimulation was effective in suppressing pain.
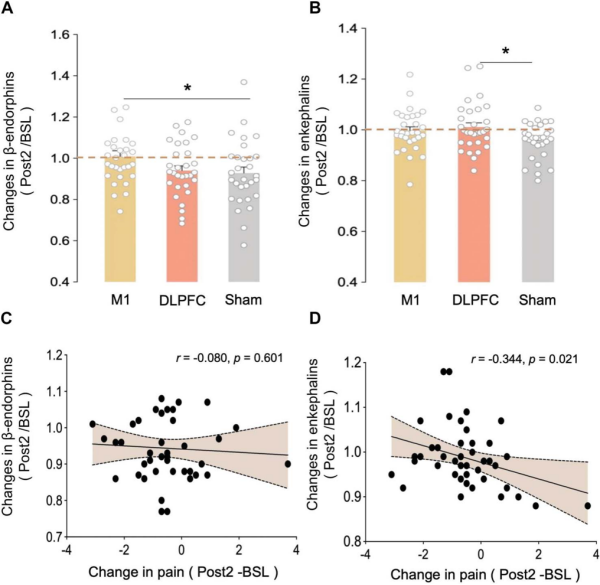
Plasma β-endorphin and enkephalin data showed that M1 stimulation significantly increased β-endorphin levels, whereas DLPFC stimulation significantly increased enkephalin levels compared with the Sham group. Correlation analysis showed that there was a covariance between the changes in enkephalins and the changes in pain intensity after pcTBS intervention.
In summary, the present study provides causal evidence for the involvement of the endogenous opioid system in the analgesic effects of M1 and DLPFC-rTMS, and further demonstrates the existence of a dose-dependent effect of this endogenous opioid peptide mechanism at different targets.
Prof. Min Yan of Zhejiang University Second Hospital was the corresponding author of this paper, and Associate Prof. Xianwei Che of Hangzhou Normal University was the co-supervisor. Dr. Ying Liu and Mr. Junfeng Sun from the Department of Anesthesiology, Second Hospital of Zhejiang University were the co-first authors. This study was also supported by Prof. Paul Fitzgerald and Prof. Bernadette Fitzgibbon of the Australian National University, and Prof. Robin Cash of the University of Melbourne. This study was supported by the National Natural Science Foundation of China (82071227), the National Key Clinical Specialties Construction Project (2021-LCZDZK-01), the Zhejiang Provincial Key Research and Development Program (2022C03038), and the Zhejiang Provincial Natural Science Foundation (LY23C090002).
Wang Y, Tan B, Shi S, Ye Y, Che X. Dopamine D2 receptor antagonist modulates rTMS-induced pain experiences and corticospinal excitability dependent on stimulation targets. Int J Clin Health Psychol. 2024;24(1):100413. doi:10.1016/j.ijchp.2023.100413
Zhao H, Jiang C, Zhao M, et al. Comparisons of Accelerated Continuous and Intermittent Theta Burst Stimulation for Treatment-Resistant Depression and Suicidal Ideation. Biol Psychiatry. Published online December 22, 2023. doi:10.1016/j.biopsych.2023.12.013
Cheng M, Che X, Ye Y, et al. Analgesic efficacy of theta-burst stimulation for postoperative pain. Clin Neurophysiol. 2023;149:81-87. doi:10.1016/j.clinph.2023.02.174
This content is organized by the Clinical Support Department of Shenzhen Yingchi Technology Co.,Ltd. Criticisms and corrections are welcome. For reprint, please indicate the source.