Release time :2022-08-08
Source:support@yingchitech.com
Scan:1455
Chronic pain is a major source of suffering. It interferes with daily functioning and often is accompanied by distress. Chronic pain is defined as pain that persists or recurs for more than 3 months by ICD-11. In chronic pain syndromes, pain can be the sole or a leading complaint and requires special treatment and care. [1]
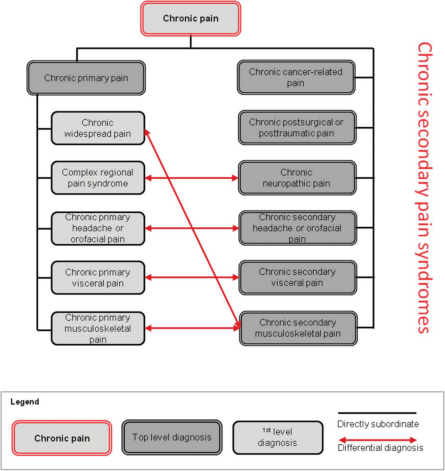
A systematic classification of chronic pain was developed by a task force of the International Association for the Study of Pain (IASP). In conditions such as fibromyalgia or nonspecific low-back pain, chronic pain may be conceived as a disease in its own right and we call this subgroup “chronic primary pain.” In 6 other subgroups, pain is secondary to an underlying disease: chronic cancer-related pain, chronic neuropathic pain, chronic secondary visceral pain, chronic posttraumatic and postsurgical pain, chronic secondary headache and orofacial pain, and chronic secondary musculoskeletal pain. These conditions are summarized as “chronic secondary pain” where pain may at least initially be conceived as a symptom.[1]
Chronic pain exerts an enormous personal and economic burden, affecting more than 30% of people worldwide according to some studies. Prevalence rates of chronic pain vary between 11% and 40%, with a study by the US Centers for Disease Control and Prevention (CDC) estimating the point prevalence at 20.4%. A systematic review comprising studies done in the UK reported a pooled chronic pain prevalence rate of 43.5%, with the rate of moderate-to-severe disabling pain ranging from 10.4% to 14.5%. A largescale 4-year longitudinal study, also done in the UK, found the annual incidence rate for chronic pain to be 8.3%, with a recovery rate of 5.4%.[2]
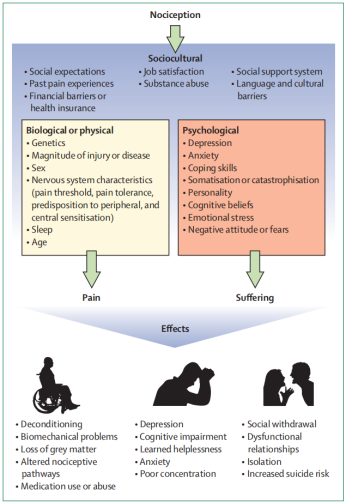
The biopsychosocial model postulates pain and disability as multidimensional, dynamic interactions among biological, psychological, and social factors that reciprocally influence each other. It is generally accepted that characteristics such as depression, anxiety, poor sleep, and adverse social conditions can be the result of chronic pain, but it is less commonly known that these factors also predispose individuals to chronic pain. Psychological factors associated with the development of chronic pain include depression, anxiety, post-traumatic stress, poor coping skills, and catastrophisation, among others. Sociocultural factors that are associated with chronic pain include low educational attainment, culture, and poor social support. Contributing biological factors include genetics, age, sex, sleep, hormones, and endogenous opiate systems .[1]
TMS is a non-invasive neuromodulation technology. The time-varying pulsed magnetic field can penetrate the skull non-invasively, act on the central nervous system, generate induced currents, and cause a series of physiological and biochemical reactions, thereby affecting metabolism and neuronal excitability in the brain so that improve and treat mental and neurological diseases.
In general, high-frequency stimulation increases the cortical excitability, whereas low-frequency stimulation has inhibitory control on the neural circuits. High-frequency rTMS (>5 Hz) produces an excitatory effect on neural tissue by inducing a form of long-term potentiation (LTP) that increases efficacy at the synapse that can last beyond the duration of a treatment session. Lowfrequency rTMS (≤1 Hz) produces an inhibitory effect on neural tissue via a long-term depression(LTD)-like mechanism.
Theta burst stimulation (TBS) is a form of TMS that also modulates neural function in a way that reflects LTP-like and LTD-like mechanisms that last longer than those induced by rTMS. TBS uses high-frequency bursts (3 pulses at 50 Hz) that are repeated at intervals of 200 milliseconds. Intermittent TBS (iTBS) increases the amplitude of neuron responses and increases cortical excitability in human motor cortex, whereas a continuous TBS (cTBS) pattern suppresses evoked responses.
Successful pain management with magnetic stimulation may rely on a combination of stimulation types and targets that are tailored to the disease and specific symptoms.[5]
Recently, the International Federation of Clinical Physiology (IFCN) published guidelines for the clinical use of TMS, listing high-frequency (HF) rTMS of the primary motor cortex (M1) contralateral to the painful side for neuropathic pain as an A level recommendation and HF-rTMS of the left M1 or DLPFC for improving quality of life or pain, respectively, in fibromyalgia as a B level recommendation,[3] and in 2018 the Chinese Physicians Association Neuromodulation Specialty Committee on Electroconvulsive and Neurostimulation published Expert Consensus on repeat Transcranial Magnetic Stimulation Therapy, which recommends the use of rTMS high-frequency stimulation of the motor area of the contralateral cortical area of pain ( M1) for chronic neuropathic pain, rTMS low-frequency stimulation of the occipital lobe for migraine (FDA approved), and rTMS high-frequency stimulation of L-DLPFC or motor cortex for non-neuropathic pain such as fibromyalgia, complex regional pain syndrome Type I.[4]
The excellent safety profile of TMS is the major advantage of this noninvasive treatment modality and led to the expansion of this therapy for a variety of indications. The most serious of the reported complications is the occurrence of seizures, and most of these complications occurred either because of nonadherence to recommended stimulation parameters or in patients who were on medications (tricyclic antidepressants, antipsychotics, theophylline, cocaine, alcohol, amphetamines, MDMA [3,4-methylenedioxy-N-methylamphetamine]) that are known to lower seizure threshold. The risk of epilepsy following TMS is <1% in healthy individuals which increases to 1.4% in patients with history of epilepsy.
In addition, TMS in patients with intracranial implants can result in heating or magnetization of these implants and impaired functioning. Therefore TMS is relatively contraindicated in patients with DBS (Deep brain stimulation) electrodes, cochlear implants, or aneurysm clips, whereas it is safer in patients with titanium implants or implants containing ferromagnetic alloys. Other reported adverse effects include histotoxicity, hearing disorders, persistent local pain, transient headache or discomfort at the TMS coil application, impaired cognition, psychiatric symptoms, and biological alterations. [5]
Reference
[1] Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19-27. doi:10.1097/j.pain.0000000000001384
[2] Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082-2097. doi:10.1016/S0140-6736(21)00393-7
[3] Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018) [published correction appears in Clin Neurophysiol. 2020 May;131(5):1168-1169]. Clin Neurophysiol. 2020;131(2):474-528. doi:10.1016/j.clinph.2019.11.002
[4] 李达, 许毅, 安建雄. 重复经颅磁刺激治疗专家共识[J]. 转化医学杂志, 2018, 007(001):4-9.
[5] Young NA, Sharma M, Deogaonkar M. Transcranial magnetic stimulation for chronic pain. Neurosurg Clin N Am. 2014;25(4):819-832. doi:10.1016/j.nec.2014.07.007