Release time :2022-12-15
Source:support@yingchitech.com
Scan:2080
Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulation technology. The time-varying pulsed magnetic field can penetrate the skull non-invasively, act on the central nervous system, generate induced currents, and cause a series of physiological and biochemical reactions, thus affecting brain metabolism and neuronal excitability, so as to improve and treat mental and neurological diseases.
TMS therapy mainly achieves the function of excitation or inhibition of the local cerebral cortex by changing the stimulation frequency, and treats diseases by bidirectionally regulating the balance between the excitation and inhibition functions of the brain.
①Non-invasive;②Painless;③Safe, with little side effects;④Reliable efficacy;⑤It can be used for clinical detection and clinical treatment.
The main stimulation modes of TMS are single-pulse transcranial magnetic stimulation (sTMS), paired transcranial magnetic stimulation (ppTMS), repetitive transcranial magnetic stimulation (rTMS) and patterned stimulation (TBS).
Single-pulse transcranial magnetic stimulation (sTMS) outputs one stimulation pulse at a time, which is mainly used for electrophysiological examination, such as measuring motor threshold (MT), motor evoked potential (MEP), central motor conduction time (CMCT), functional area mapping, etc.
Paired transcranial magnetic stimulation (ppTMS) outputs two pulses in pairs at a time, and the interval between the two pulses is adjustable from 0 ms to 100 ms. According to whether the two pulses are output by the same stimulation coil, stimulate the same part in pairs or use two stimulation coils to stimulate different parts successively in pairs, it can be divided into single-beat paired stimulation and double-beat paired stimulation. It is mostly used to detect the excitability and inhibition of cortical nerves, conduction and functional integrity between cortices.
At present, two modes of conventional repetitive transcranial magnetic stimulation (rTMS) and patterned stimulation (TBS) are mostly used in clinical therapy.
Stimulation can be released continuously after a single instruction. Slow rTMS (1Hz or slower) or fast rTMS (greater than 1Hz) can be given at the same stimulation site. The fastest speed can reach 100 times per second, that is, 100Hz. The longest continuous stimulation time of a single sequence can be up to 10s, which is widely used (as shown in the figure below).
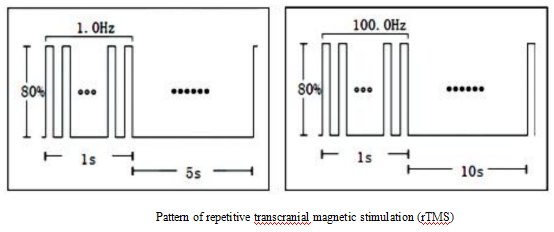
Generally speaking, high-frequency stimulation increases cortical excitability, while low-frequency stimulation inhibits cortical excitability. High-frequency rTMS (≥ 5 Hz) can induce long-term potentiation (LTP) effects and produce excitatory effects on neural tissue. Low-frequency rTMS (≤ 1Hz) can induce long-term depression (LTD) effects and inhibit neural tissue.
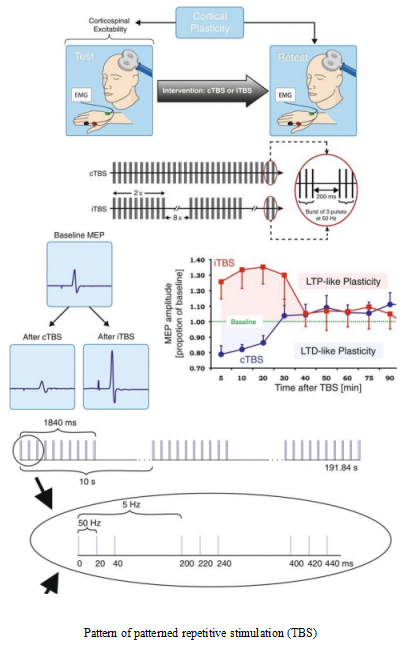
The sequence of patterned stimulation is quite different from that of conventional repetitive stimulation. Patterned stimulation adds the pattern of clump-like or cluster-like stimulation. For example, 3 pulses form a clump or cluster, which is equivalent to that each clump or cluster is a pulse of conventional repetitive stimulation, and multiple clump stimuli are combined into a string of stimulation.
It has three modes, iTBS, imTBS and cTBS. iTBS can induce long-term potentiation effects and excite the cortex. imTBS is equivalent to stimulation between 1-5Hz, which has little effect on cortical excitability, so it is generally not used. cTBS can induce long-term depression effects and inhibit cortical excitability (as shown in the figure below).
TMS can be used for the treatment and diagnosis of diseases. In terms of disease diagnosis, TMS is used as a clinical research tool to assess cortical processing time, cortical connectivity, cortical inhibition, excitability, and cortical interactions. In terms of clinical treatment of diseases, TMS mainly excites or inhibits local cerebral cortex functions by changing the stimulation frequency, and treats diseases by bidirectionally regulating the balance between brain excitation and inhibition functions. It is mainly used for the treatment of a variety of psychiatric, neurological, neurological rehabilitation and children's brain diseases.
The clinical applications of repetitive transcranial magnetic stimulation in psychiatry mainly include: depression, anxiety, obsessive-compulsive disorder, schizophrenia, bipolar disorder and post-traumatic stress disorder. Clinical applications in the field of neurology mainly include: Parkinson's disease, Alzheimer's disease, consciousness disorder, epilepsy, multiple sclerosis (lower limb spasm), etc. Clinical applications in the field of rehabilitation mainly include: post-stroke dyskinesia, post-stroke aphasia, dysphagia, lateral neglect, fibromyalgia and so on. The clinical applications in the field of children's rehabilitation mainly include: autism, cerebral palsy, attention deficit hyperactivity disorder, Tourette's syndrome, language retardation, etc.